News Archive for April 2015
Simpleware ScanIP software receives FDA 510(k) market clearance
April 23, 2015
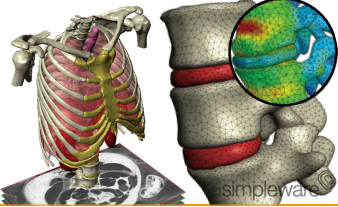
UK-based Simpleware’s 3D image processing software ScanIP has received 510(k) marketing clearance from the U.S. Food and Drug Administration. ScanIP is intended for use as a software interface and image segmentation system for the transfer of imaging information from a medical scanner such as a CT scanner or an MRI scanner to an output file. […]
- July 2019
- February 2019
- December 2018
- November 2018
- October 2018
- August 2018
- July 2018
- June 2018
- May 2018
- April 2018
- March 2018
- February 2018
- January 2018
- December 2017
- November 2017
- October 2017
- September 2017
- August 2017
- June 2017
- May 2017
- March 2017
- February 2017
- January 2017
- November 2016
- October 2016
- September 2016
- July 2016
- June 2016
- May 2016
- April 2016
- March 2016
- February 2016
- January 2016
- December 2015
- November 2015
- September 2015
- July 2015
- June 2015
- May 2015
- April 2015
- March 2015
- February 2015
- January 2015
- December 2014
- November 2014
- October 2014
- September 2014
- August 2014
- July 2014
- June 2014
- April 2014
- March 2014
- February 2014
- January 2014
- December 2013
- October 2013
- September 2013
- February 2013