Innovation in orthopaedic medical device manufacture Right product, right process, right patient, right time
Latest News
Innovate UK commissions new survey on impact of new EU Medical Device Regulations for UK SMEs
July 26, 2019
Innovate UK commissions survey to understand how new EU Medical Device Regulations, due to be fully implemented on 26 May 2020, will impact UK SMEs. Read more …
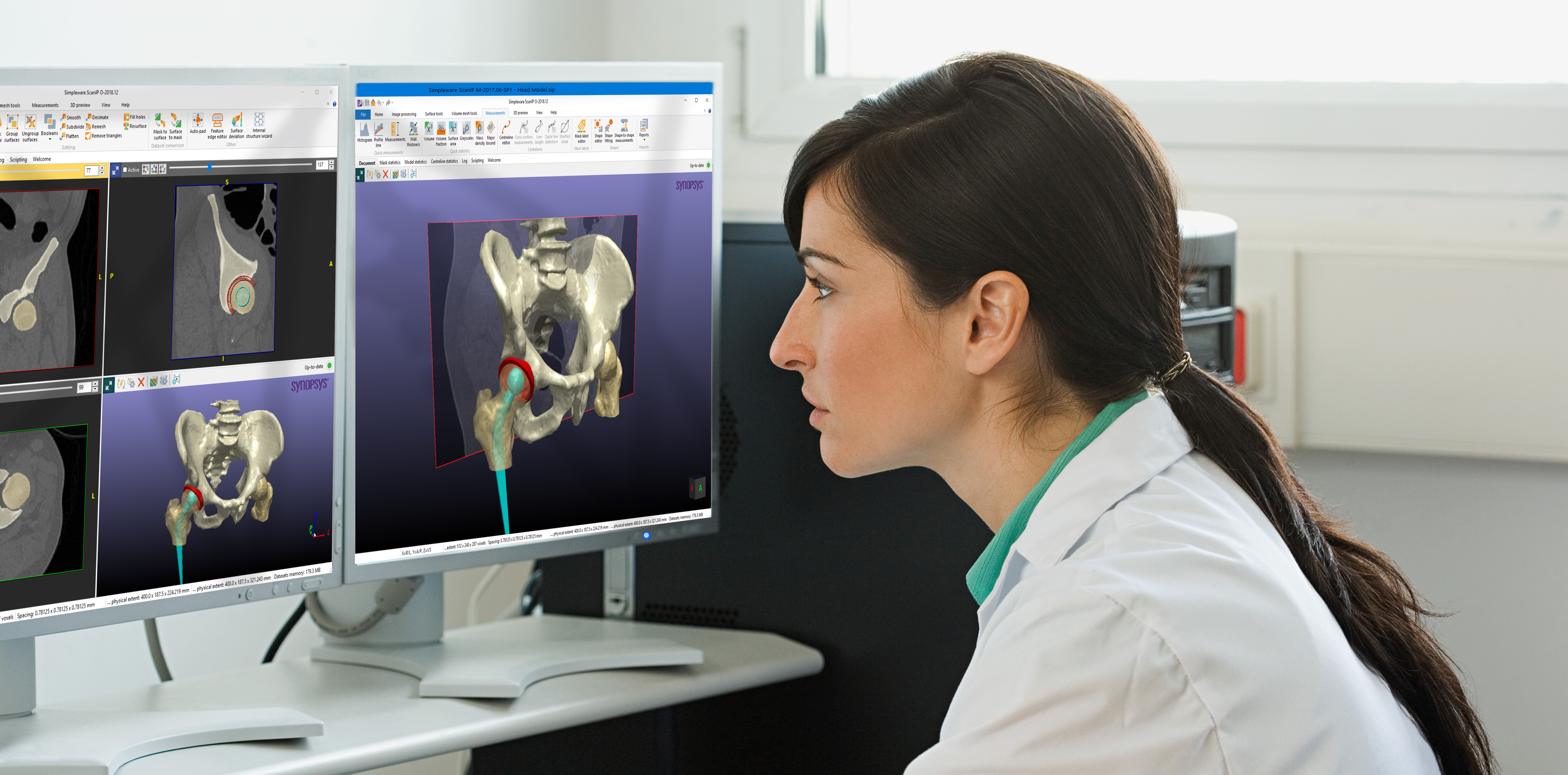
Partner news: Solutions for Clinical Applications with Simpleware ScanIP Medical
February 12, 2019
Synopsys are pleased to announce the release of the Synopsys Simpleware™ ScanIP Medical edition. This exciting new version of Simpleware ScanIP comes with FDA 510(k) clearance, and CE and ISO 13485:2016 certification as a medical device. Simpleware ScanIP Medical is the ideal choice for those working with 3D imaging to create medical devices for pre-clinical […] Read more …
Partner news: Improved Usability and Data Processing with Synopsys Simpleware Software Release O-2018.12
December 12, 2018
Synopsys, Inc. today launched Simpleware™ Release O-2018.12. The latest version of Simpleware software includes many exciting new features and performance improvements. Version O-2018.12 significantly enhances Simpleware’s ease-of-use and reliability as a comprehensive solution for 3D image processing and analysis workflows. Simpleware software is an established solution for users wanting to build high-quality models from […] Read more …
Partner news: EnviroGlass batch reformulation presents significant cost savings and environmental benefits
November 27, 2018
Leading specialists in glass, Glass Technology Services Ltd, have demonstrated that reductions in CO2 emissions, combined with significant cost savings, may be possible for glass manufacturers through batch reformulation. Carried out in partnership with Sheffield Hallam University, the EnviroGlass project proposed that substantial savings may be possible and has successfully demonstrated proof of concept […] Read more …
Upcoming Events
Ask the Expert: Clinically-led manufacture of custom-made collagen membranes
October 30, 2018
Location: Barts and the London School of Medicine and Dentistry, London
Ask the Expert: Clinically-led manufacture of custom-made collagen membranes Tuesday 30th October 2018, 10:00 – 15.30 Seminar room, 4th Floor, Barts and the London School of Medicine and Dentistry, Turner Street, Whitechapel, London, E1 2AD This event will highlight key performance requirements and future needs for the scalable manufacture of next-generation collagen-based biomaterials from the […]
The Leeds Orthopaedic Biomechanics course 2019
January 21, 2019
The Institute of Medical & Biological Engineering at the University of Leeds is offering a specialist 2 day short course on Orthopaedic Biomechanics on 21 and 22 January 2019. The course aims to give delegates an understanding of the necessary fundamentals of biomechanics and how they are applied to solve problems in orthopaedics. This course […] Read more …
Search Our National Capability Database
Click here to search our complete database of manufacturing capabilities
Latest Blog Posts
Ligament repair – Case study
August 21, 2018
Injuries to the anterior cruciate ligament (ACL) are a common sporting injury, resulting in loss of stability in the knee. They account for around 40% of all sports injuries and in the USA over 70,000 ACL reconstructions are performed annually. Most procedures use autograft transplants from a healthy tendon elsewhere in the patient’s body while a smaller number use donor tissue from a deceased donor. Read more …
Bryan Stuart: Life after MeDe Innovation
July 3, 2018
After five years as one of our Early Career Researchers, Bryan secured a role at the University of Oxford. Here he discusses his time with MeDe Innovation: As I move into the next stage of my career I reflect on the incredible opportunities that MeDe Innovation offered over the last 5 years, during my […] Read more …